H2so4 Naoh Balanced Equation
2 2KOH H2SO4 K2SO4 2H2O Chapter 10 Acids Bases and Salts. You can use parenthesis or brackets.

How To Balance Naoh H2so4 Na2so4 H2o Youtube
P4O10 H2O -- H3PO4 What is the numerical coefficient in A.

. Label Each Compound With a Variable. A student carries out a titration to find the concentration of 650ml of HCl using 0250M NaOH. Balance the equation NaOH H2SO4 Na2SO4 H2O using the algebraic method.
The balanced equation will appear above. C the sum of the coefficients of the reactants is equal to the sum of the coefficients of the products. Use uppercase for the first character in the element and lowercase for the second character.
Label Each Compound With a Variable. Fe Au Co Br C O N F. Ordinary Differential Equations.
Fe Au Co Br C O N F. Balance the following chemical equation. Lets say a molecule HNO3 is having a normality of 05.
Equation of a plane distance of a point from a plane condition for coplanarity of three lines angles between two planes angle between a line and a plane. Titration is the process which help to determine. The reaction was performed using 45 g NaHCO3 and 18 g NaOH were produced.
Label each compound reactant or product in the. Balance the equation Fe H2SO4 Fe2SO43 H2 using the algebraic method. What is the formula mass in atomic mass units for H2SO4.
For NaOH valency is one as it gives one OH- ion when it dissolves in water. Compound states like s aq or g are not required. A chemical equation can be balanced as follows by using algebraic method.
How To Calculate Limiting Reagents. For Sulphuric acid H2SO4 the valency is 2 as you get 2 H ions when you dissolve it in water. B the total number of ions is the same in reactants and products.
For HCl Hydrochloric acid valency is one because when you dissolve it in the water you get one H ion. Once you know how many of each type. Use uppercase for the first character in the element and lowercase for the second character.
CopperII oxide is added a little at a time to the acid until no more can dissolve. Label each compound reactant or product in the. A chemical equation is balanced when A the total number of molecules is the same in reactants and products.
77 Best place and safest website to buy cheap Ruined King CurrencyRPRiot Points Top Up service for PCPS4Xbox One discount price ever biggest promotions. To balance NaOH HCl NaCl H2O youll need to be sure to count all of atoms on each side of the chemical equation. Water H2O can act as.
For example C6H5C2H5 O2 C6H5OH CO2 H2O will not be balanced but XC2H5 O2 XOH CO2 H2O will. Balancing of the equation means that Number of reactant side atom. CuO H2SO4 CuSO4 H2O Step 2 Filter off the excess copperII oxide using a filter paper and funnel.
Order and degree of a differential equation formulation of a differential equation whole general solution is given variables separable method. The balanced equation will appear above. Determine a balanced equation for the reaction of molecular nitrogen N2 and oxygen O2 to form dinitrogen pentoxide.
NaOH HCl NaCl H2O Eg. Replace immutable groups in compounds to avoid ambiguity. A balanced chemical equation and ii an ionic.

Sodium Hydroxide Sulfuric Acid Acid Base Neutralization Reaction Youtube

Type Of Reaction For H2so4 Naoh Na2so4 H2o Youtube
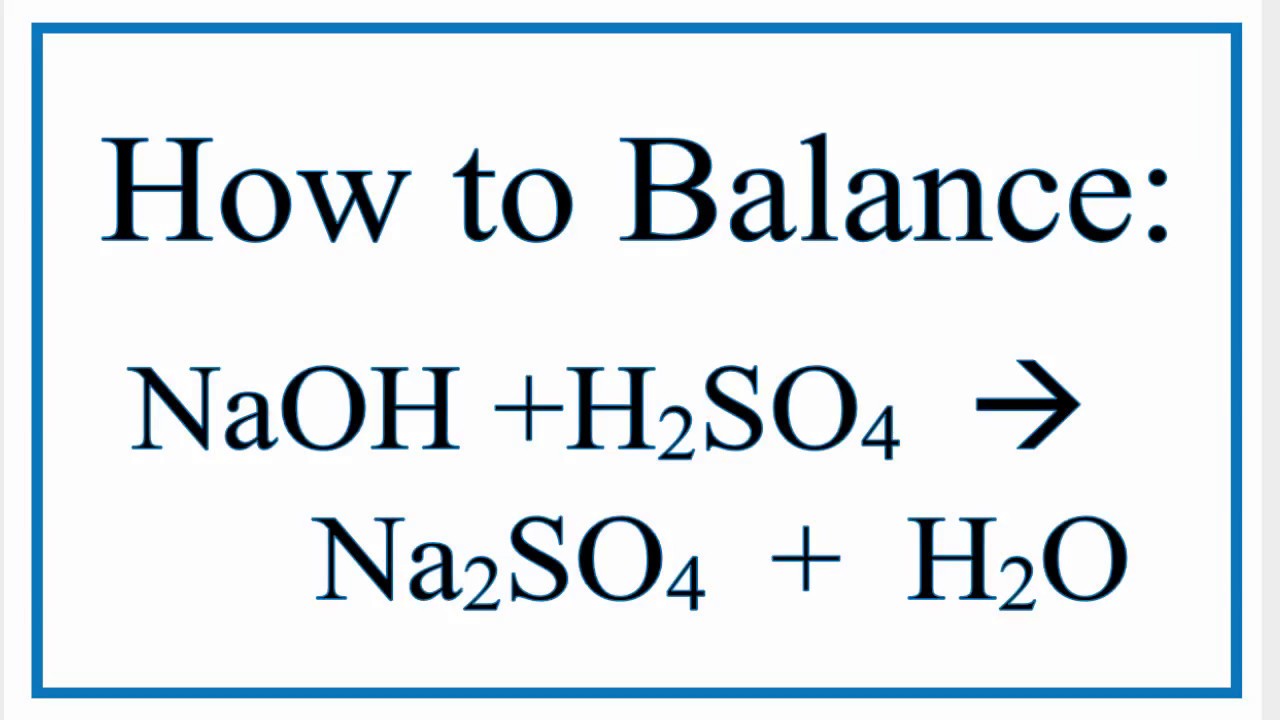
How To Balance Naoh H2so4 Na2so4 H2o Youtube
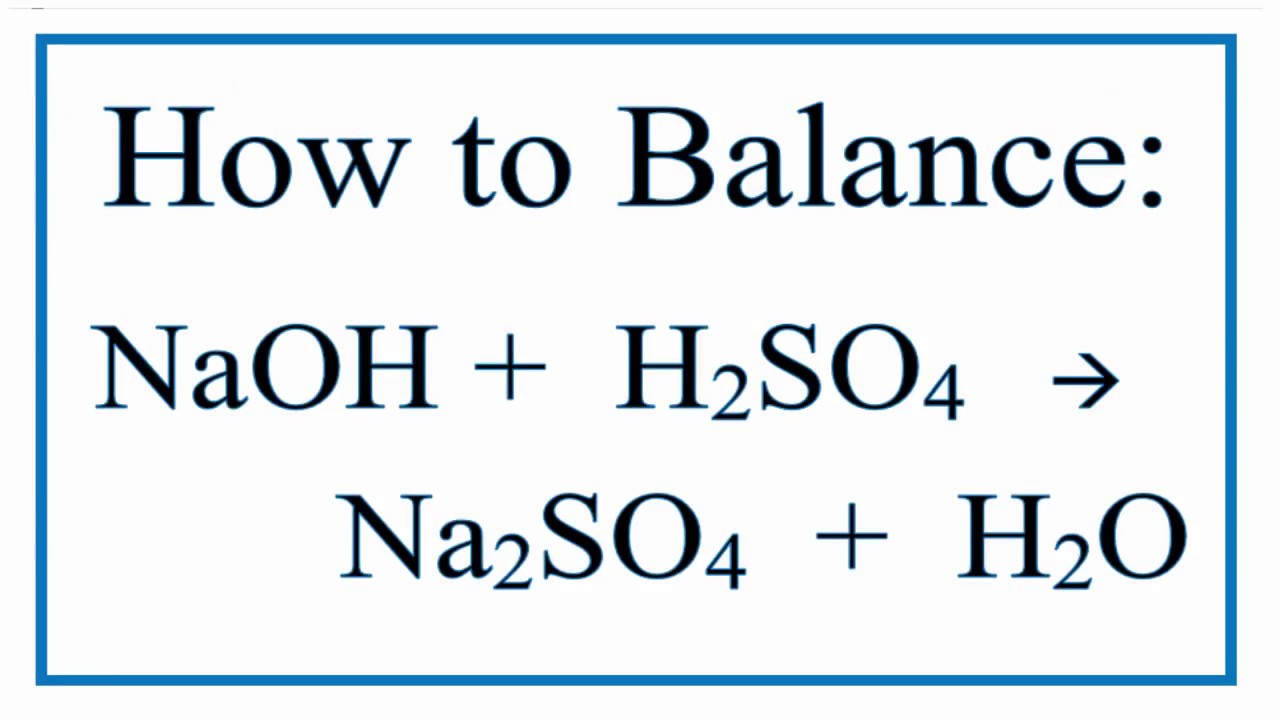
Sodium Hydroxide And Sulfuric Acid Yields Sodium Sulfate And Water Youtube
Comments
Post a Comment